Data Integrity - How to deal with stricter requirements

What is „Data Integrity“?
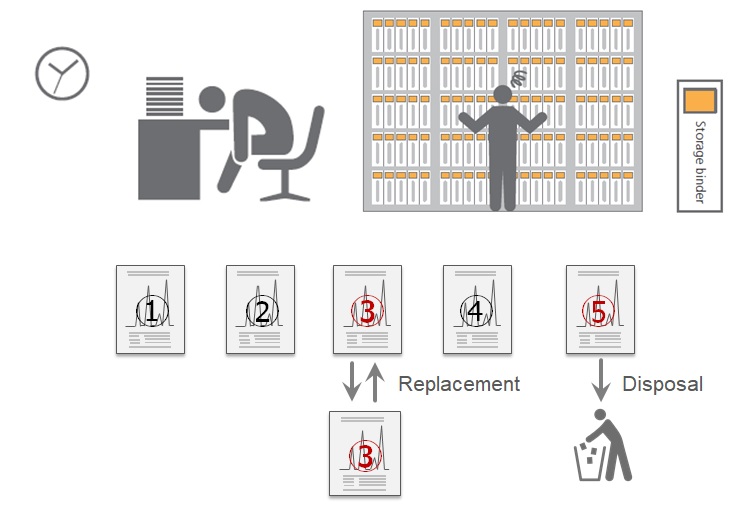
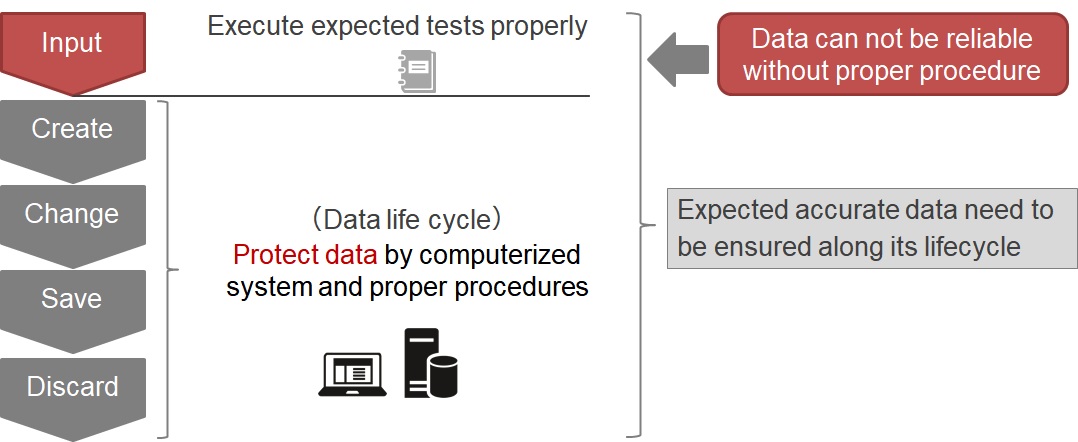
Data Integrity is defined as the extent to which all data are complete, consistent and accurate throughout the “data lifecycle”.
All data need to be protected and managed not to be tampered with throughout the data LifeCycle.
To ensure reliability of acquired data, operating procedures have to be followed and computerized systems need to protect data Integrity.
Webinar
Assuring Laboratory Data Integrity in a Time of Enhanced Regulatory Oversight
ISO/IEC 17025:2017
New general requirements for the competence of testing and calibration laboratories
To maintain high quality of analytical results from accredited laboratories and better ensure data integrity in the light of new technological developments, a new version of the ISO 17025 standard was published. The new ISO 17025:2017 has been in effect since November 2017. Every accredited laboratory is now obligated to be assessed according to the new version by the end of 2020, in order to keep their status of accreditation.
Data integrity is a key requirement of ISO/IEC 17025:2017
A secure and future-oriented software solution is required to fulfill the new requirements with regard to technical records, electronic signatures and data management.
For any lab already involved with GxP, terms such as compliance, ALCOA+, GAMP, PIC/S and 21CFR Part 11 are very well known. With the changes made in ISO 17025:2017 these principles mostly required in the pharmaceutical industry, will also become increasingly important for testing and calibration laboratories. Shimadzu offers a comprehensive solution - not only for chromatography systems, but also for spectrophotometers and other laboratory instruments. Users can securely integrate a large number of systems under uniform user administration to be prepared for the new requirements.