OPTIC-4 - Sollicitaties
Multimode Inlet for Gas Chromatograph-Mass Spectrometer

Most of the documents on the LITERATURE is available in PDF format. You will need Adobe Acrobat Reader to open and read PDF documents. If you do not already have Acrobat Reader, you can download it free at the Adobe's Website. Click the GET ADOBE READER icon on the left to download a free copy of Adobe Acrobat Reader.
1. Content of 1,4-Dioxane in Shampoo
Suspected of being carcinogenic, 1,4-dioxane is sometimes found as an impurity in cosmetic products. The use of the DMI mode for the quantitation of 1,4-dioxane in shampoo was investigated. A cryo-trap was used in order to make the peaks sharper. By optimizing the temperature of the injection port, none of the high-boiling-point impurities in the shampoo, which can cause contamination of the column, were introduced to the column, and 1,4-dioxane was quantitated with a simple pretreatment. This mode makes use of thermal extraction and is useful in reducing the amount of pretreatment that is needed.
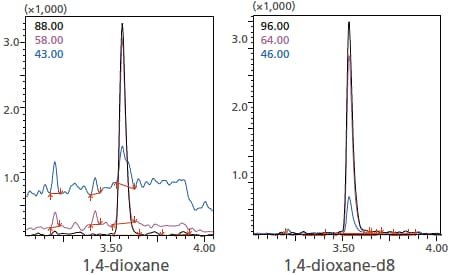
A SIM chromatogram resulting from shampoo to which 1,4-dioxane was added so as to produce a concentration of 3.6 ppm is shown in the figure at left. In the figure at right, the SIM chromatogram of 4-dioxane-d8 used for quantitation is shown. The result of quantitation was 3.6 ppm.
2. Odor from Clothing
In dealing with household odors, it is important to analyze the components of the odor. Using the MonoTrap thermal desorption mode, the volatile components of socks immediately after being worn were analyzed. A sock that had just been removed by the wearer was placed in a sample bag along with the MonoTrap for sampling. A cryo-trap was used in order to make the peaks sharper. By using this mode, the volatile components of clothing, such as odors, can be concentrated and detected with high sensitivity, through simplified procedures.

Total ion current chromatogram
1=Hexanal
2=Heptanal
3=Octanal
4=6-Methyl-5-heptene-2-one
5=Nonanal
6=Dichlorobenzene
7=Dodecanal
8=Decanal
9=Propylene Glycol
10=trans-Geranylacetone
11=Muskalactone
12=Benzyl salicirate
3. Odor from Product
In order to solve problems related to odors, it is necessary to identify the substance that is causing it. Using the MonoTrap thermal desorption mode, the substance at the source of the disinfectant smell emanating from resin-based parts in an electrical device was identified. Some material was scraped from the chassis emitting the odor, and was placed inside a vial together with MonoTrap, and the odorous component was extracted and concentrated. As the substance at the source of the odor, 2,6-dibromophenol (2,6-DBP), which has a low odor threshold, was detected. By using this mode, even components having a low odor threshold can easily be concentrated and detected.

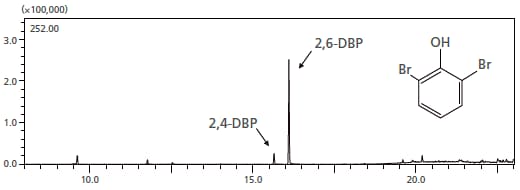
Mass chromatogram
4. Pyrolysis of Resin
Pyrolysis gas chromatography is effective for the structural analysis of resins. In pyrolysis gas chromatography, it is necessary to rapidly heat the sample so that the pyrolysis products do not take part in a second-order reaction. Since this system is capable of rapid heating to temperatures of up to 600 °C, at a speed of 60 °C/s, it is capable of providing data that is equivalent to that produced by instantaneous-heating pyrolyzers. Using this mode, polycarbonate resins were analyzed. Numerous phenolic compounds, including bisphenol A, were detected. The results were virtually identical to those yielded by instantaneous-heating pyrolyzers.
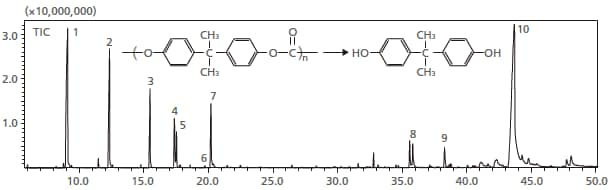
Pyrolysis total ion chromatogram of polycarbonate resin
1=phenol
2=p-cresol
3=p-ethylphenol
4=p-vinylphenol
5=p-isopropylphenol
6=p-tert-butylphenol
7=p-isopropenylphenol
8=p-hydroxy-2,2-diphenylpropane
9=p-hydroxy-3-methyl-2,2-diphenylpropane
10=bisphenol A
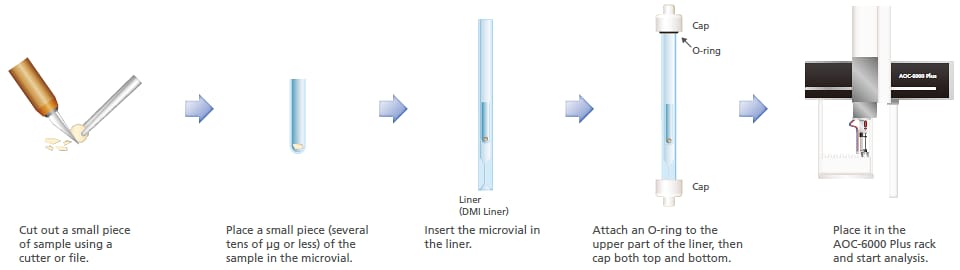
5. Reactive Pyrolysis of Resin
Reactive pyrolysis GC-MS (thermal assisted hydrolysis and methylation GC/MS; THM-GC/MS) is effective for the structural analysis of resin samples that produce polar compounds as a result of thermal decomposition. THM-GC/MS performs alkaline hydrolysis while heating the sample, methylates the product to form derivative compounds, and carries out measurement using GC/MS. This system is capable of THM in an inert glass microvial. Using this mode, polycarbonate resins were analyzed. Of the two hydroxyl groups of the bisphenol A produced by hydrolysis, one was methylated in a compound detected, and two were methylated in another compound.
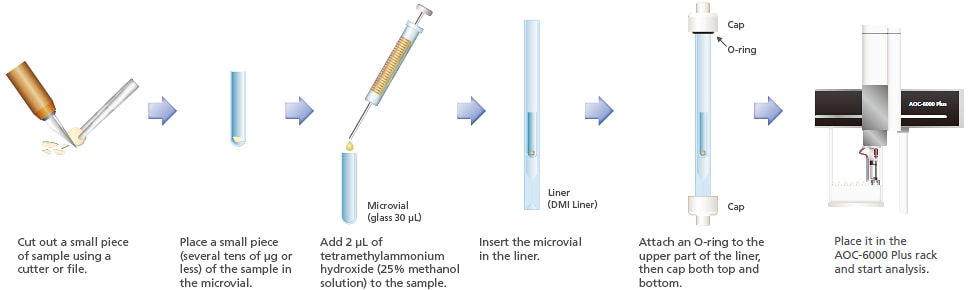
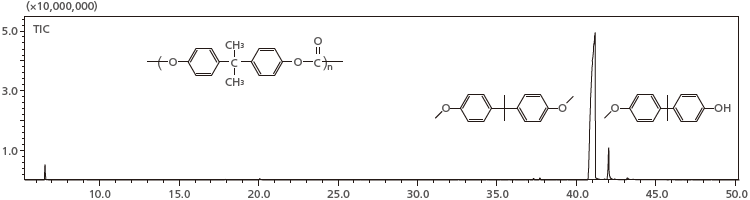
Reactive pyrolysis total ion chromatogram of polycarbonate resin
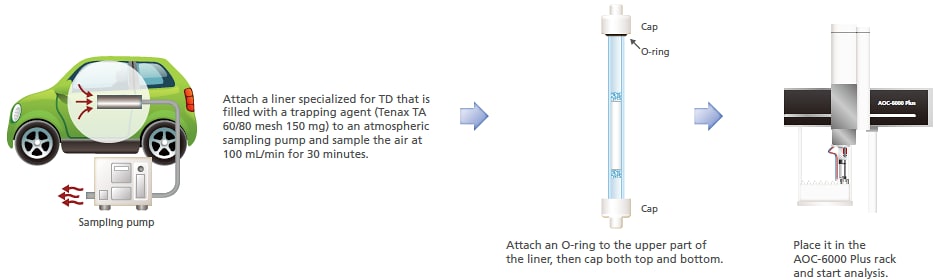
6. Atmospheric Gas in Automobile
In consideration of the quality of the environment within an automobile, efforts to reduce the volatile organic compounds (VOCs) inside an automobile are ongoing. VOCs inside an automobile were analyzed using solid adsorption-thermal desorption. A liner filled with a trapping agent was exposed to the air inside an automobile. Afterwards, this system was used to heat the liner and analyze the desorbed components. A cryo-trap was used in order to also target low-boiling-point components. Detected substances included toluene, ethyl benzene, and xylene. Also detected were dibutyl phthalates, which were vaporized as a result of direct sunlight heating resins.
This mode can be effectively used to analyze trace components in gases.
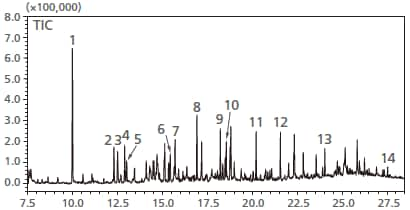
Total ion chromatogram of atmospheric air in an automobile
Total ion chromatogram of atmospheric air
in an automobile
1=Toluene
2=Ethylbenzene
3=m-,p-Xylene
4=Styrene
5=o-Xylene
6=p-Dichlorobenzene
7=2-Ethyl-1-hexanol
8=Nonanal
9=Menthol
10=Decanal
11=Tridecane(C13)
12=Tetradecane(C14)
13=Hexadecane(C16)
14=Di-n-butyl phthalate(DBP)
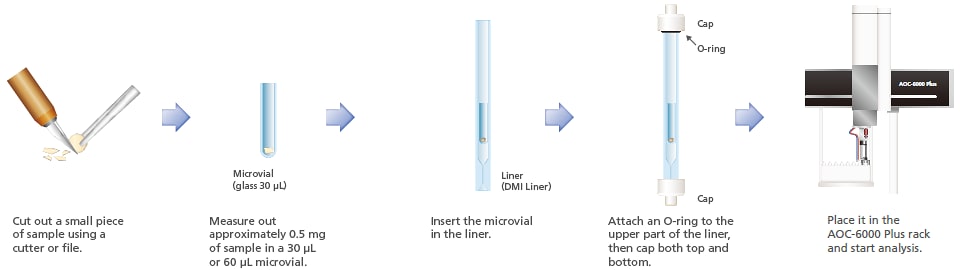
7. Additives in Resins
RoHS directives restrict the use of four phthalate esters; diisobutyl phthalate (DIBP), dibutyl phthalate (DBP), butyl benzyl phthalate (BBP), and di(2-ethylhexyl)phthalate (DEHP). As pretreatments for analysis of phthalate esters in resins, thermal extraction is used for screening, and solvent extraction-liquid injection is used for accurate quantitation. Resin samples were analyzed using DMI-thermal extraction. This system automatically switches between thermal extraction and liquid sample injection methods. As a result, screening and accurate quantitation can be performed without troublesome system switchovers.
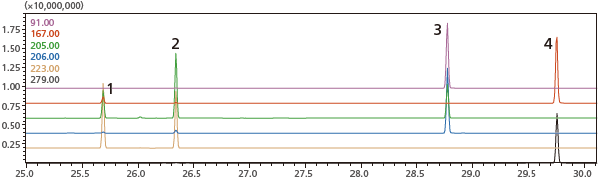
1=DIBP
2=DBP
3=BBP
4=DEHP
8. Aroma from Food Products
Aroma is an important factor in giving foods an appealing taste. Using MonoTrap and adsorbing elements using PDMS, volatile components from blue cheese were collected and analyzed using thermal desorption. Compared to adsorbing elements using PDMS, the MonoTrap yielded a greater number of detected peaks, and the analysis provided greater sensitivity. By using this mode, trace components from food products, such as aromas, can be detected with high sensitivity.
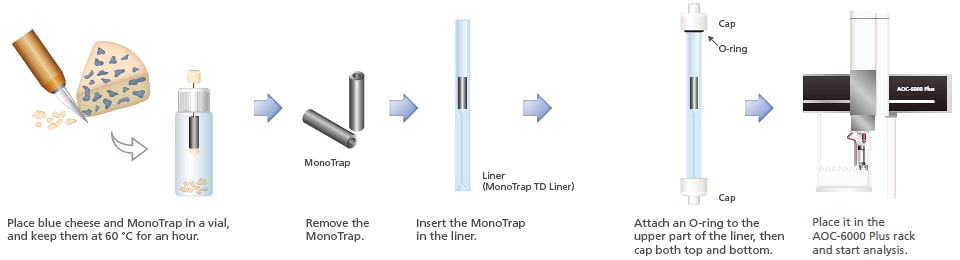
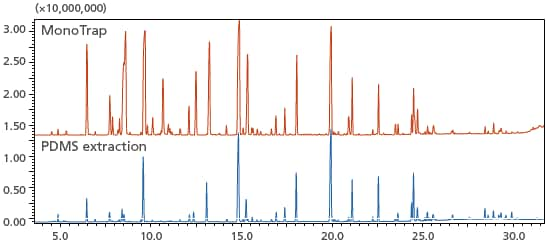
Total ion chromatograms of volatile components of blue cheese acquired through
concentrations using MonoTrap (above) and PDMS extraction (below)


