The Nexera series is a family of HPLC systems that marries AI and IoT enhancements to set new industry standards.
Shimadzu Retention Time Fluctuations - Part 2
6 - Changes in Peak Shape - Part 2
9 - Retention Time Fluctuations - Part 1
10 - Retention Time Fluctuations - Part 2
11 - Column Lifespan - Part 1
12 - Column Lifespan - Part 2
13 - Detector Issues
14 - Flow Line Leakage
15 - Course Summary
In the last section, we looked at the influence of temperature and fluctuating flow rates on retention time. In this course, we will look at the influence of eluents on retention time. If the composition or density of the mobile phase changes, it quickly leads to retention time fluctuations.
1. Inadequate Re-Equilibration in the Gradient
 |
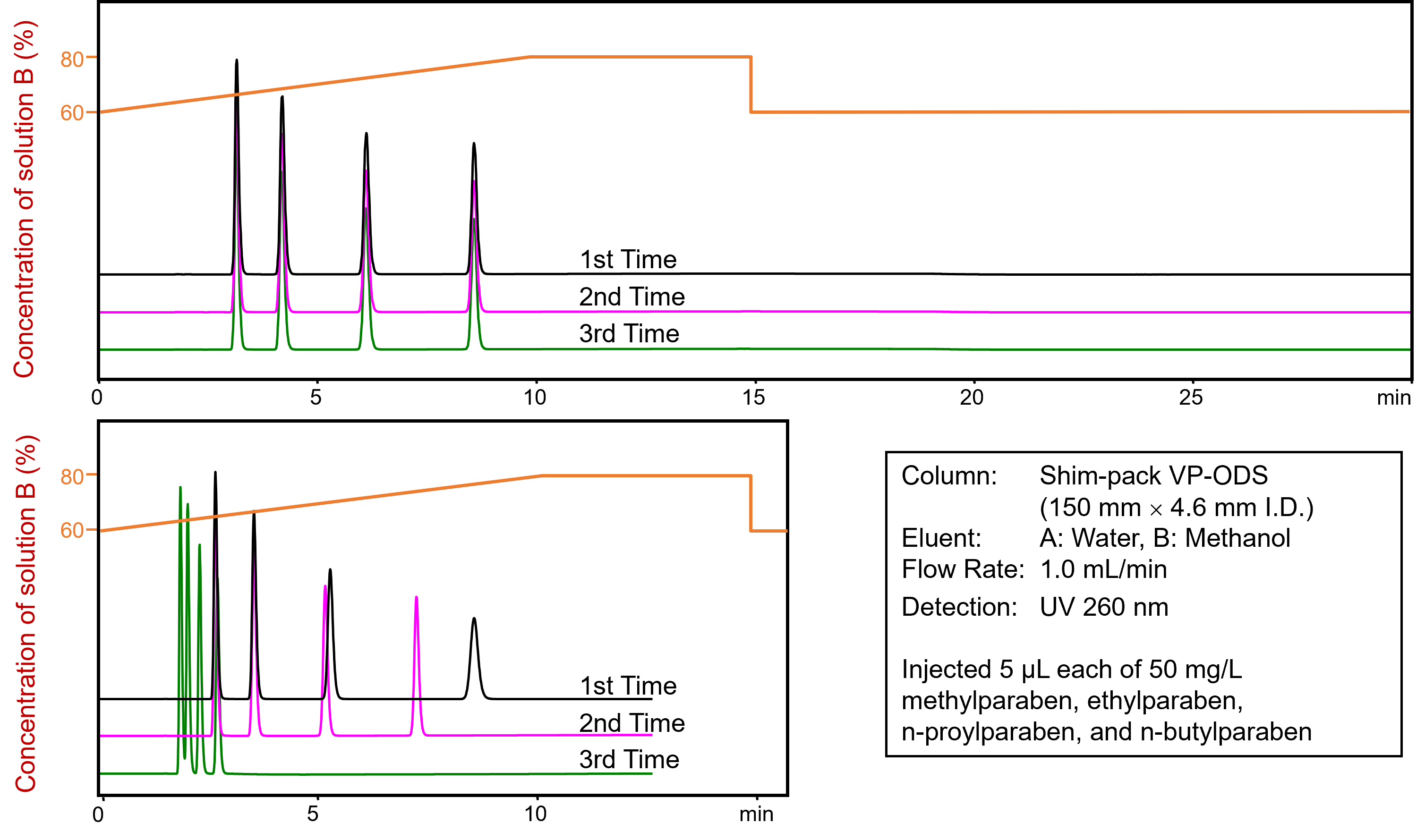
At the beginning of each measurement, it is important to ensure that the column is in equilibrium. When switching between two different mobile phases, such as when washing out the storage solvent, the column must be equilibrated with sufficient column volumes (typically 10 column volumes for reversed phase chromatography). This also applies to methods using a gradient as it is important to equilibrate the column back to the initial conditions of the run. Commonly, reverse phase chromatography methods have a long rinse step with strong (high organic) solvent to wash any strongly retained analytes off the column. To ensure that the strong solvent doesn't impact the next run, a sufficiently long equilibration step that corresponds to the initial conditions of the method is required.
If the equilibration is too short, it can lead to retention time fluctuations or, as shown in the example above, progressively shorter retention times from measurement to measurement due to the strong solvent still on the column. In this case, the column is no longer in equilibrium. |
2. Change in Eluent Composition
 |
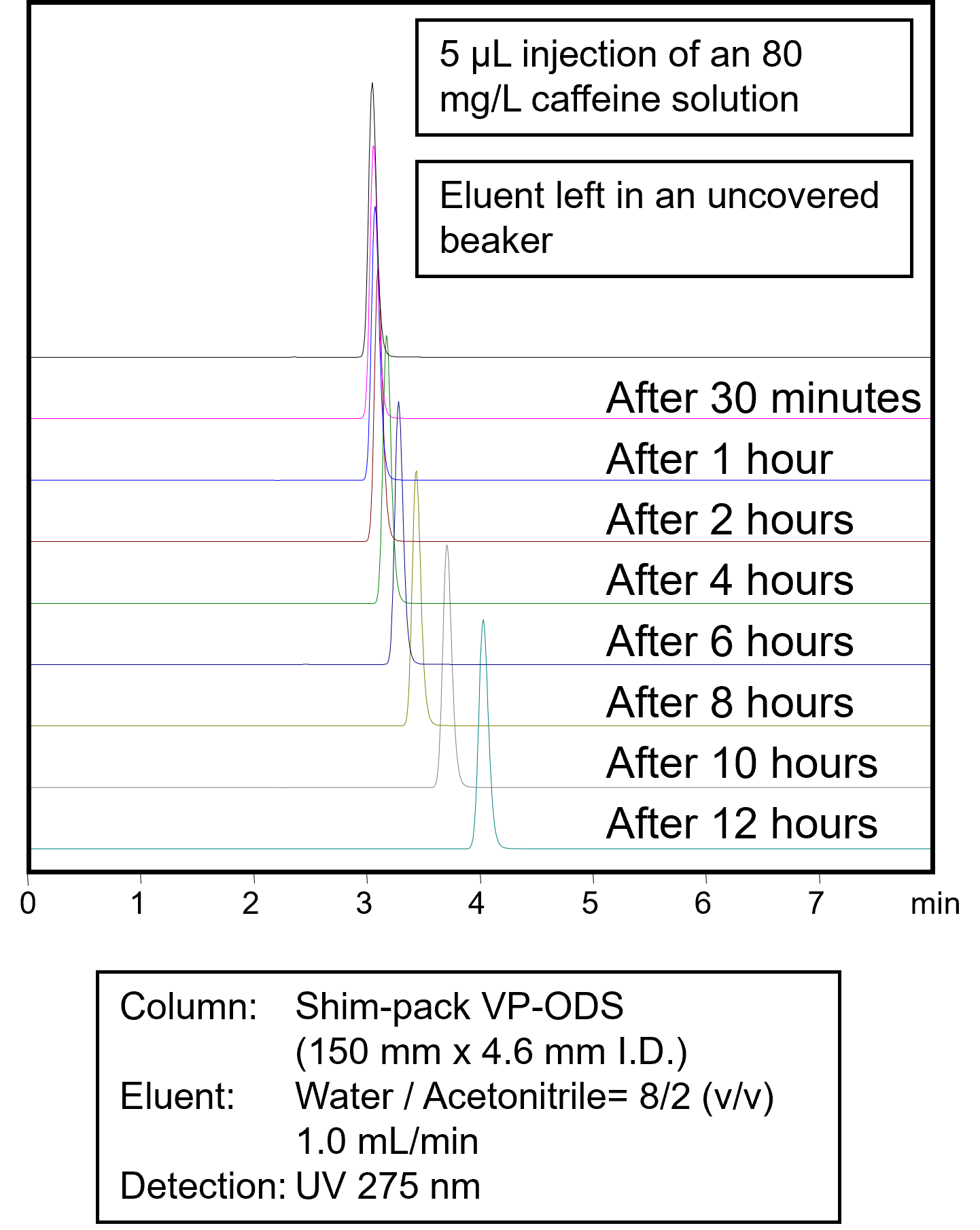
Great attention should be paid to the mobile phase. Together with the column, the eluents are crucial for separation and stable measurements. Therefore, store your eluents in suitable storage bottles and seal them with appropriate tight-fitting caps. Do not leave the eluents in the sun or in the direct draft of an air conditioner. Depending on the composition of the eluent, some of the organic solvent can quickly evaporate, changing the composition of the eluent. In the image to the right, you can see the influence of leaving an unsealed eluent bottle containing a mixture of water and acetonitrile. The more acetonitrile evaporates, the lower the elution strength, and the longer the retention times. |
 |
3. Preparation of Eluents
 |
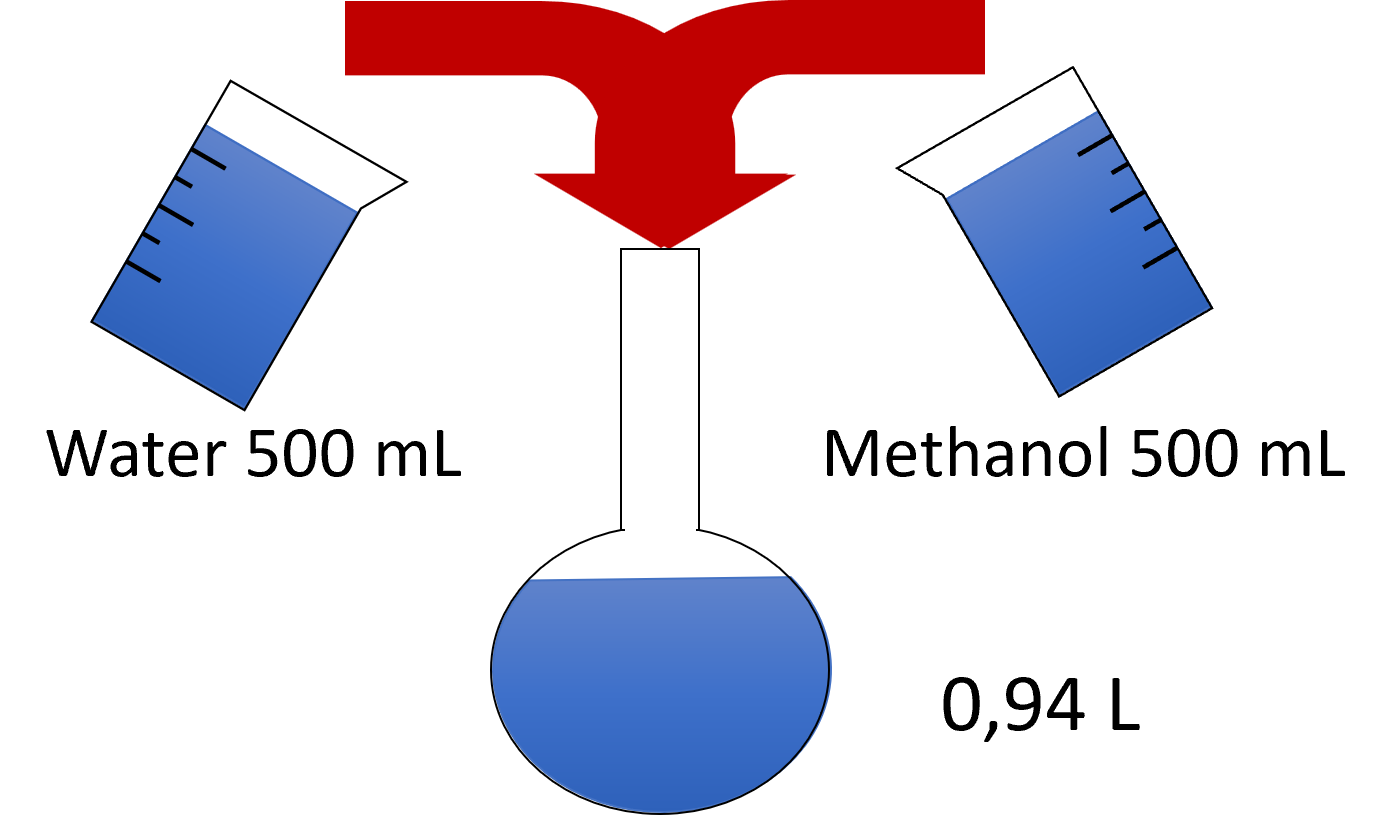
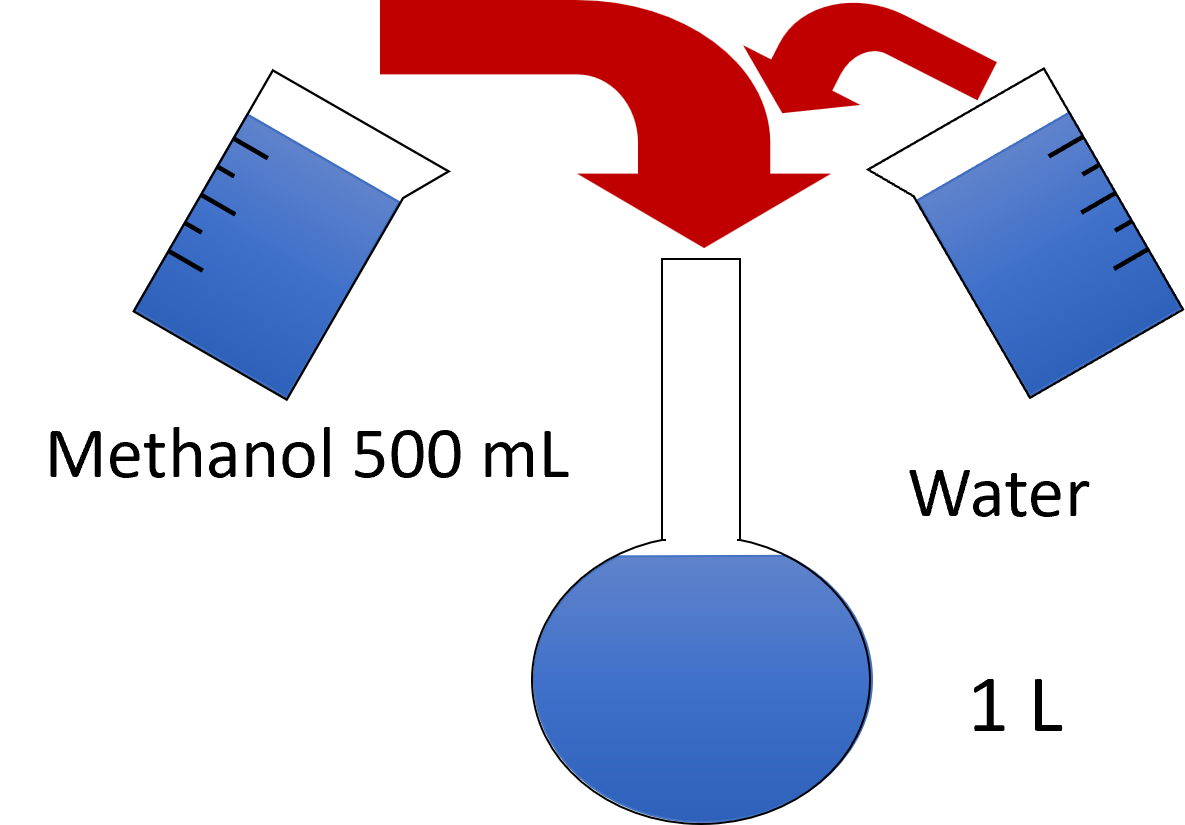
Often, issues with mobile phases arise from poor eluent preparation. You should develop precise procedures to ensure consistent quality of the eluents. A good example is the preparation of a 50% methanol solution in water. This solution can be prepared in different ways, and depending on which employee prepares it, different results can occur. |
 |
|
|
Employee 1:
|
Employee 2:
|
||
|
These two solutions have different elution strengths, as seen in the following example with a phosphate buffer and acetonitrile. The retention times of the individual analytes fluctuate significantly and, in the worst case, can fall outside the identification time window.
For this reason, some users choose to purchase pre-mixed mobile phases to ensure consistency of their eluents.
|
|||
 |
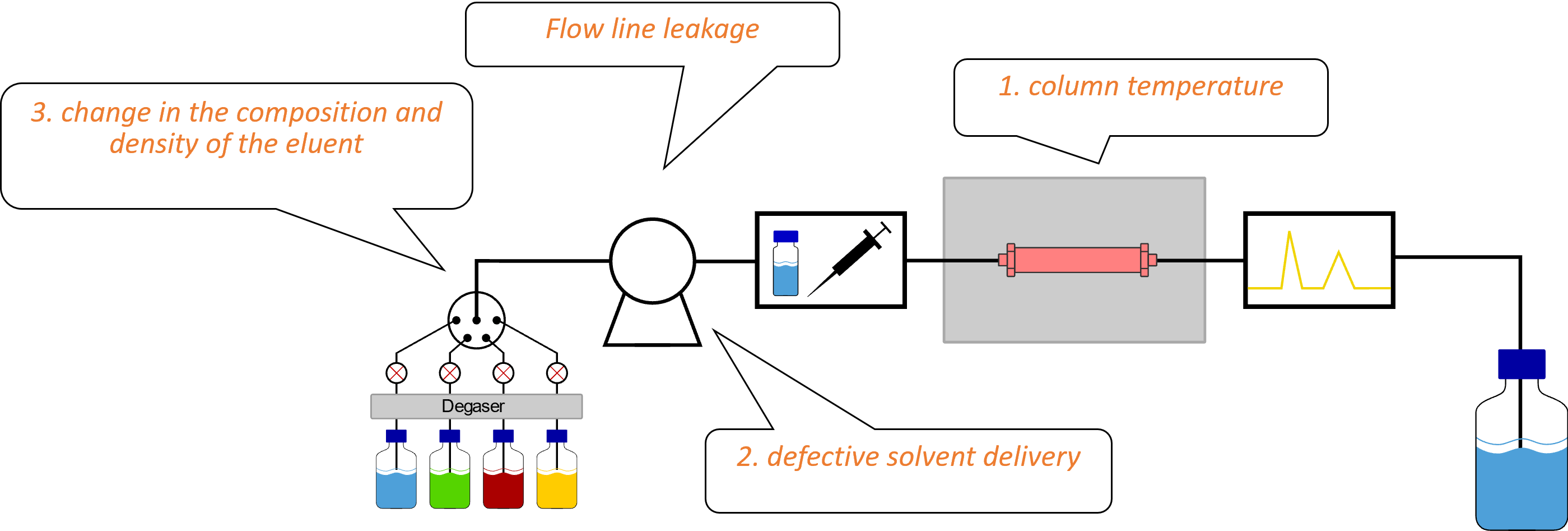
In addition to the reasons listed here, there are many other reasons that can cause retention time fluctuations. These can include insufficient buffer capacity, poor online mixing, a defective or dried-out column, or accumulating contaminants. If you are dealing with fluctuating retention times, it is recommended to first check the temperature control of the column and then check the pump and the eluents. For a quick test, replace the eluents with new ones and check the retention times with your references. Using the instrumentation to create these kinds of mixtures will ensure maximum accuracy.
|
||

Things to consider daily:
Problematic Conditions:
- Reversed-phase eluent is 100% water
- Extremely acidic or alkaline eluents in silica columns
- Normal-phase chromatography
- Reverse-phase chromatography with ion-pair reagents
General:
- Keep a column diary
- Document maintenance
- Develop procedures for the preparation of eluents
- Store storage bottles sealed and safely
- Pay attention to sufficient equilibration time
The upcoming two course units will focus on the separation column and its lifespan.
Your Shimadzu LC Team
Related Resources
-
-
Nexera UHPLC