Software & Informatics
Software & Informatics
Shimadzu Software & Informatics
Shimadzu introduced its first digital integrator in 1969. Since then, Shimadzu has been a leader in the field of processing chromatographic data. The Shimadzu laboratory software portfolio features a range of high-quality software solutions to fit any laboratory’s requirements and streamline your workflow.
In the pharmaceutical industry, compliance to regulations and guidelines, such as CSV and PIC/S GMP, U.S. FDA 21 CFR Part 11, and data integrity, as well as the proper, efficient management of instruments and analytical data is crucial. To survive in this environment, faster and more efficient management of instruments and data is essential. Our LabSolutions analytical data management software enables compliance with data integrity requirements by providing a system for managing various analytical instruments and test information in a single location.
Learn more about our full lineup of software and informatics products below.
Software & Informatics – Frequently Asked Questions
What is LabSolutions?
LabSolutions is Shimadzu’s unified software platform for instrument control, data acquisition, processing, reporting, and regulatory compliance. It supports LC, LCMS, GC, GCMS, UV‑Vis, FTIR, AA, ICP, and more within a single environment.
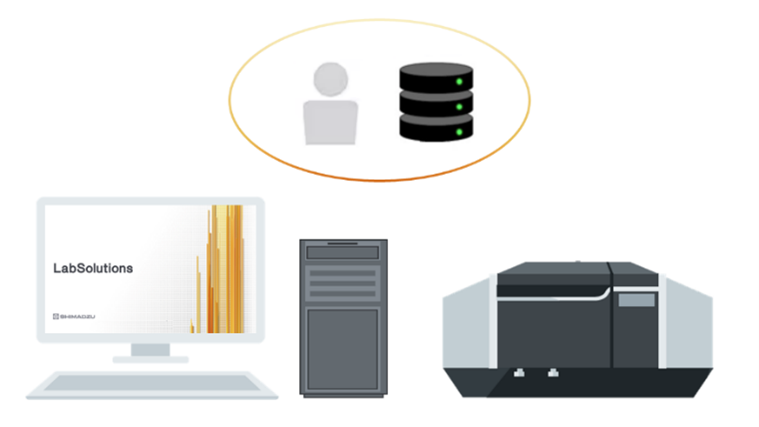
What are the different LabSolutions configurations?
LabSolutions Workstation: Stand‑alone PC for local instrument control and data processing.
LabSolutions DB / DB LCMS: Centralized database on a single PC for secure data management.
LabSolutions CS: Network‑based, server‑hosted solution allowing multi‑instrument, multi‑user access and full compliance management.
Does the software support regulatory compliance?
Yes. LabSolutions includes comprehensive compliance features such as audit trails, user access control, electronic signatures, data integrity tools, and secure database storage to meet 21 CFR Part 11 and EU Annex 11 requirements.
What is LabSolutions MD?
LabSolutions MD is Shimadzu’s automated method development software, enabling gradient optimization, multi‑column screening, SFC method development, and intelligent method comparison. It significantly accelerates chromatographic optimization.
Can LabSolutions integrate multiple techniques?
Yes. LabSolutions allows unified control of LC, GC, LCMS, GCMS, UV‑Vis, FTIR, AA, ICP, and Material Testing systems in one platform, simplifying training and improving workflow consistency across the laboratory.
Does Shimadzu provide automated sample handling software?
Yes. For LCMS automation, the CLAM (Clinical Laboratory Automated Module) platform integrates with LabSolutions for hands‑off sample preparation, automated workflows, and minimized human error.
Does the software include tools for data review and reporting?
LabSolutions provides template‑based reporting, batch review, automated peak integration, spectral matching, quantitative workflows, and multi‑data overlays for comparison studies.
Is remote access or network deployment available?
Yes. LabSolutions CS offers centralized data storage, browser‑based access, and secure network operation across departments or multiple laboratories. It supports mixed instrument fleets and multi‑user environments.
What informatics tools support mass spectrometry?
LCMS workflows benefit from specialized modules including peak extraction, MRM optimization tools, library search, quantitative templates, and high‑resolution mass analysis tools for QTOF systems.